Supercritical water conditions
The fourth state of water, known as supercritical, is reached at temperatures and pressures above 374°C and 220 bar. To achieve supercritical water heat is added to a closed system and temperature and pressure is increased up to supercritical conditions.
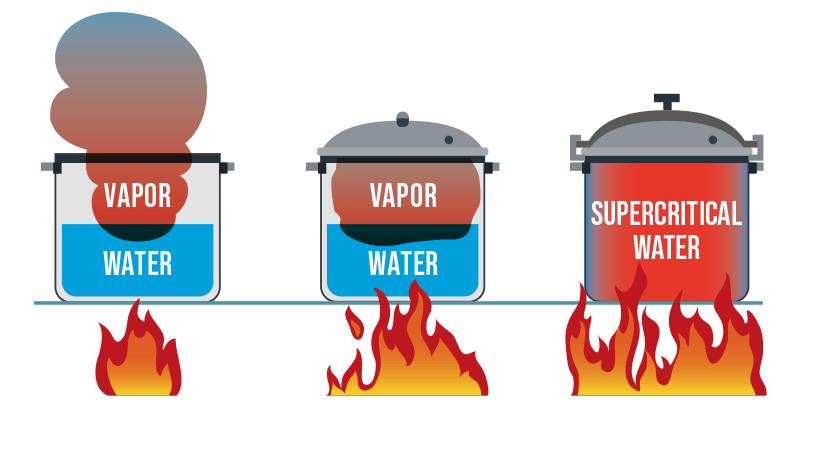
Image from https://renmatix.com/understanding-supercritical-water
Physicochemical and thermochemical properties of supercritical water are significantly different from those in liquid or gaseous state:
- Higher dissociation constant: this allows greater autoionization into hydronium and hydroxide ions.
- Supercritical water behaves like a nonpolar substance: this produces that the water become highly miscible with nonpolar gases such as H2 and O2, avoiding the formation of bubbles.
- Increases the ionic strength of water: this is translated to high diffusion coefficients that decrease mass transfer limitations and facilitate efficient chemical transport, as the transfer of OH– from the cathode to the anode.
X-SEED takes advantage of supercritical water properties, such as absence of bubbles, high conductivity, high diffusion coefficients, and modification of dissociation constant to decrease the internal resistance and improve voltage efficiency. The presence of KOH or other salts in water also affects the behavior and properties of supercritical water, and thus is a key research point of the project.
The X-SEED electrolyzer will operate with Supercritical water conditions, which means at least 374 ºC and 220 bar. Operating the electrolyzer at high temperatures allows for both kinetics and thermodynamic benefits, such as increasing voltage efficiency, current density, and raising the H2 productivity and process efficiency (thus reducing electricity consumption). High pressure operations, on the other hand, allow to deliver H2 at high pressures reducing or eliminating the need for post-electrolysis compression.
Advantage of high temperature
Low temperature electrolyzers, such as PEMEL, requires amount of voltage (Eop) to achieve high current and high H2 production rates. This voltage is substantially higher than Eth, resulting in energy waste in the form of heat, causing high energy demand for H2 production (> 55 kWh/kg H2), it means low energy efficiency. If electrolysis is performed at high temperature, as X-SEED electrolyzer, and the heat consumed is provided externally; the operative voltage, Eop, is highly decreased and as consequence the energy efficiency is raised, able to achieve < 48 kWh/kgH2.
Advantage of high pressure
H2 has a high energy density per mass compared to methane (120 MJ/kg H2 versus 50 MJ/kg CH4). However, its volumetric energy density is lower (10.8 MJ/m3 H2 versus 35.8 MJ/m3 CH4) due to low density of H2. Hence, high-pressure compression of H2 is required to transport and/or utilize it. Alkaline and PEMEL electrolyzers typically operate at pressures lower than 30 bar. However, high-pressure operation poses challenges such as increased gas crossover and safety considerations. Despite this, an electrolyzer that delivers H2 at high pressure offers clear advantages. On one hand, it eliminates or greatly reduces the energy demand and the cost required for post-production compression of H2 for high-pressure delivery. On the other hand, compressing liquid water is less energetically expensive than compressing gaseous H2. Regardless, X-SEED analyses the gain to increase the water pressure to deliver H2 at high pressure instead of post compression of generated H2 at low pressure.
References to go further:
Aulakh, Deepinder Jot Singh, Kiari Goni Boulama, and Jon G. Pharoah. “On the reduction of electric energy consumption in electrolysis: A thermodynamic study.” International Journal of Hydrogen Energy 46.33 (2021): 17084-17096.
Gupta, Sahil, et al. “Developing new heat-transfer correlation for supercritical-water flow in vertical bare tubes.” International Conference on Nuclear Engineering. Vol. 49309. 2010.
Flarsheim, William M., et al. “Electrochemistry in near-critical and supercritical fluids. 3. Studies of bromide, iodide, and hydroquinone in aqueous solutions.” The Journal of Physical Chemistry 90.16 (1986): 3857-3862.
Boll, H., E. U. Franck, and H. Weingärtner. “Electrolysis of supercritical aqueous solutions at temperatures up to 800 K and pressures up to 400 MPa.” The Journal of Chemical Thermodynamics 35.4 (2003): 625-637.
Borsboom-Hanson, Tory, Thomas Holm, and Walter Mérida. “Techno-economics of sub-and supercritical water electrolysis.” Energy Conversion and Management 265 (2022): 115741.
Borsboom-Hanson, Tory, Thomas Holm, and Walter Mérida. “A high temperature and pressure framework for supercritical water electrolysis.” International Journal of Hydrogen Energy 47.48 (2022): 20705-20717.
Holm, Thomas, et al. “Hydrogen costs from water electrolysis at high temperature and pressure.” Energy Conversion and Management 237 (2021): 114106.



